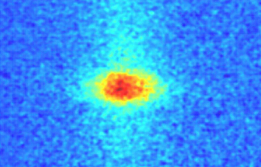
Molecules containing three atoms were first cooled by laser to ultra-low temperature. This was done by John Doyle and colleagues at Harvard University, who used a cooling technique called Sisyphus to cool about one million strontium hydroxide molecules to 750μK. The team said the work opened the way for a series of applications, including quantum simulation and precision measurements.
With the blue laser pointer to the atomic gas cooled to ultra-low temperature, first appeared in the late 1970s, completely changed the material quantum state of the study. The first Bose-Einstein condensate was manufactured in the laboratory in 1995. The first Fermi-Dirac condensate was created in 2003 as two important milestones for this technology. The technique relies on the fact that the photon carries a small amount of momentum, and under certain conditions, the atomic reabsorption and re-emission of the photon can reduce its random motion and thus reduce its temperature.
The rotation of molecules and the freedom of vibration make the cooling of molecules (not atoms) more complicated, which affects their absorption and emission of photons. Thus, the absorption and emission of photons can cause the molecules to enter a “dark state” that is no longer involved in the cooling process. Despite the many challenges, David DeMille and colleagues at Yale University still tried to use 30mw green laser to cool strontium fluoride diatomic molecules in 2014.
In this latest work, John Doyle and colleagues at Harvard University have now cooled the three-atom hydroxyl strontium molecules, named after the Greek hero Sisyphus, who was forced to push a boulder onto the top of the hill, Roll down and then often resemble this work forever. Sisyphus cooling process is the molecule through the “climb” by the burning laser standing waves generated by the potential energy loss of kinetic energy process.
When they spontaneously transition the state that no longer interacts with the light, the atoms reach the “peak”. At this point, the applied magnetic field causes the atoms to return to their original state – ready to climb again. This process is repeated many times, each cycle will reduce the kinetic energy of the atom, thereby reducing their random motion and temperature.
The key to the success of the Doyle team is that the cooling process is achieved quickly within 100μs, involving only about 200 photons interacting with each molecule. This speed is crucial because the molecule is unlikely to enter the dark state before the end of the cooling.
Doyle and colleagues write that their technology can also be used to cool larger and more complex strontium-based polyatomic molecules, such as substituting methyl groups for hydroxides. If the technique can be further extended to chiral molecules, it can also be used to study why some biological processes favor dextral or lethal molecules.